| |
Blanche Schwappach
Dr. rer. nat. 1996 at the Center for Molecular Neurobiology in Hamburg. Postdoctoral work at the University of California, San Francisco. Group leader at the ZMBH since 2000.
Assembly and trafficking of ion channels
Previous and Current research
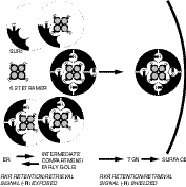
Each cell type possesses an exquisitely regulated set of plasma membrane proteins. It is essential that these be expressed in their properly folded and correctly assembled form in appropriate numbers at the cell surface. This is particularly true for ion channels since a very small number of channel proteins can have dramatic effects on the electrical excitability of a cell. Almost all ion channels are multimeric protein complexes and the respective composition of the complex can greatly affect its functional properties. How is assembly and precise stoichiometry of ion channels controlled? What determines the subcellullar localization of ion channel subunits at different stages of assembly? We have started to address some of these questions using the ATP-sensitive potassium channel as a model system (KATP channels couple the metabolic state of the cell to membrane excitability). We found that the primary quality control mechanism during KATP assembly involved the exposure of an ER localization signal present in cytosolic domains of each subunit.
We wish to understand the arginine-based ER localization signals that we identified in KATP channels as well as the cellular machinery that recognizes them. To this end we have embarked on a random screen of ER trafficking motifs in mammalian cells. The screen is based on retroviral gene transfer and flow cytometry. Eventually, we would like to ask how the cell regulates the number of channels at the cell surface. How are the relevant quality control and trafficking processes regulated in response to environmental change?Projects for graduate students
Characterization of proteins that interact with certain ion channel subunits in mammalian cells, mapping of interaction domains, regulation of interaction
Screening of expression libraries in mammalian cells to identify proteins that regulate surface expression of ion channel complexesContact:
Blanche Schwappach
ZMBH, Universitaet Heidelberg,
Im Neuenheimer Feld 282, D-69120 Heidelberg, Germany
phone +49-6221-54 68 98
fax +49-6221-54 58 94
email b.schwappach@zmbh.uni-heidelberg.de
http://www.zmbh.uni-heidelberg.de/Schwappach/
Selected publications
- Lloyd S.E., Pearce S.H.S., Fisher S.E., Steinmeyer K., Schwappach B., Scheinman S.J., Harding B., Bolino, A., Devoto M., Goodyer P., Rigden S.P.A., Wrong O., Jentsch T.J., Craig I.W., and Thakker R.V. (1996). A common molecular basis for three inherited kidney stone diseases. Nature, 379, 445-449.
Hechenberger M., Schwappach B., Fischer W.N., Frommer W.B., Jentsch T.J., and Steinmeyer K. (1996). A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. J. Biol. Chem., 271, 3363233638.
Schwappach B., Stobrawa S., Hechenberger M., Steinmeyer K., and Jentsch T.J. (1998). Golgi Localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J. Biol. Chem., 273, 1511015118.
Zerangue N. *, Schwappach B. *, Jan Y.N., and Jan L.Y. (1999, *these authors contributed equally). A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels, Neuron 22, 537-548.
Schwappach, B. *, Zerangue, N. * Jan Y.N., and Jan L.Y. (2000), *these authors contributed equally). Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron 26, 155-167.